| Mike's Pages |
Links |
Structures and Thermochemistry of the Alkali Metal Monoxide Anions, Monoxide Radicals, and Hydroxides
Benjamin Mintz, Bun Chan, Michael B. Sullivan, Thomas Buesgen, Anthony P. Scott, Steven R. Kass, Leo Radom and Angela K. Wilson
Center for Advanced Scientific Computing and Modeling (CASCaM), Department of Chemistry, University of North Texas, Denton, Texas 76203-5070, School of Chemistry and Centre of Excellence for Free Radical Chemistry and Biotechnology, University of Sydney, Sydney, NSW 2006, Australia, Research School of Chemistry, Australian National University, Canberra, ACT 0200, Australia, and Department of Chemistry, University of Minnesota, Minneapolis, Minnesota 55455-0431
Publication Date (Web): August 3, 2009
J. Phys. Chem. A, 2009, 113, 9501-9510.
ABSTRACT:
The geometries, enthalpies of formation (ΔHof), separations
of electronic states, electron affinities, gas-phase acidities, and bond dissociation energies associated with the
alkali metal monoxide anions (MO−), monoxide radicals (MO•), and hydroxides (MOH)
(M = Li, Na, and K) have been investigated using single-reference and multireference variants of the WnC
procedures. Our best estimates of the ΔHof values for the ground states at 298 K
are as follows: 8.5 (3Π LiO−), 48.5 (2Π LiO•),
−243.4 (1Σ+ LiOH), 34.2 (3Π NaO−), 86.4
(2Π NaO•), −190.8 (1Σ+ NaOH), 15.1
(1Σ+ KO−), 55.9 (2Σ+ KO•),
and −227.0 (1Σ+ KOH) kJ mol−1. While the LiO•
and NaO• radicals have 2Π ground states, for KO•,
the 2Σ+ and 2Π electronic states lie very close in energy, with our
best estimate being a preference for the 2Σ+ state by 1.1 kJ mol−1
at 0 K. In a similar manner, the ground state for MO− changes from 3Π for
LiO− and NaO− to 1Σ+ for KO−. The
1Σ+ state of KO− is indicated by the calculated
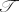 1 diagnostic and the SCF contribution to the
total atomization energy to have a significant degree of multireference character. This leads to a difference of
more than 100 kJ mol−1 between the single-reference W2C and multireference W2C-CAS-ACPF and
W2C-CAS-AQCC estimates for the 1Σ+ ΔHof for KO−.
1 diagnostic and the SCF contribution to the
total atomization energy to have a significant degree of multireference character. This leads to a difference of
more than 100 kJ mol−1 between the single-reference W2C and multireference W2C-CAS-ACPF and
W2C-CAS-AQCC estimates for the 1Σ+ ΔHof for KO−.
DOI: 10.1021/jp9034826