| Mike's Pages |
Links |
A HREELS and DFT Study of the Adsorption of Aromatic Hydrocarbons on Diamond (111)
Hui Ying Hoh, Ti Ouyang, Michael B. Sullivan, Ping Wu, Milos Nesladek and Kian Ping Loh
Department of Chemistry, National University of Singapore, 3 Science Drive 3, Singapore 117543 Institute of High Performance Computing, 1 Fusionopolis Way, #16-16 Connexis, Singapore 138632 University Hasselt, Wetenschapspark 1, B3590 Diepenbeek, Belgium
Publication Date (Web): November 5, 2009
Langmuir, 2010, 26, 3286-3291.
ABSTRACT:
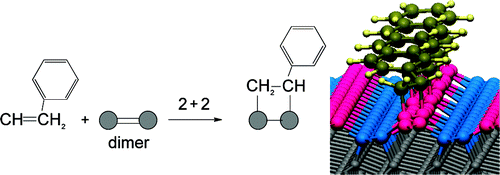
Ultrathin layers of organic molecules can be assembled on group IV (e.g., silicon, germanium, diamond) semiconductor surfaces using surface analogues of cycloaddition reactions. We present a study of the chemisorption of benzene, toluene, and styrene on the Pandey chain of C(111) using high resolution electron energy loss spectroscopy and density functional theory calculations. Two cycloaddition reactions, namely, the [4 + 2] and [2 + 2], were examined. The [4 + 2] reaction is found to be thermodynamically unfavorable on C(111), while the [2 + 2] reaction involving the ring is slightly exothermic. In the case of aromatic molecules with an external unsaturated functional group, the reaction can proceed via the external functionality, thereby preserving the aromatic ring and providing further stability. Different reactivity patterns to the C(100) surface are rationalized on the basis of steric effects imposed by the geometrical structure of the Pandey chain. Our study demonstrates the potential of employing the Pandey chain as a template for assembling one-dimensional molecular structures on the diamond surface.
DOI: 10.1021/la9030359