| Mike's Pages |
Links |
Thiophene-Containing Pechmann Dye Derivatives
Tyler B. Norsten, Eric Assen B. Kantchev, and Michael B. Sullivan
Institute of Materials Research and Engineering, A*STAR, 3 Research Link, Singapore 117602, and Institute of High Performance Computing, A*STAR, 1 Fusionopolis Way #16-16, The Connexis, Singapore 138632
Publication Date (Web): September 24, 2010
Org. Lett., 2010, 12, 4816-4819.
ABSTRACT:
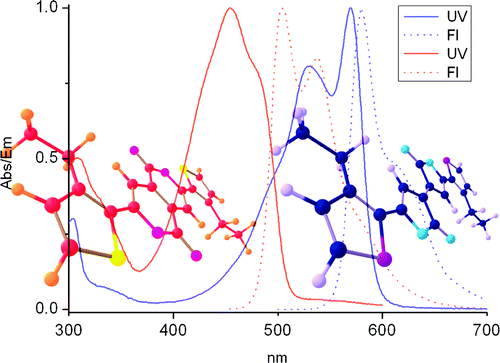
Thiophene-containing Pechmann dyes (unsaturated exo-5,5-dilactones) were easily prepared by Cu-catalyzed dehydration of the corresponding ?-aroylacrylic acids. Introduction of long alkyl chains greatly enhances solubility. Isomerization of an alkyl-substituted thiophene Pechmann dye (TPD) gave the corresponding endo-6,6-dilactone. The redox and electronic properties of these new dyes were investigated by CV, UV/vis, and fluorescence spectroscopy and DFT and TD-DFT computations. Strong absorption and emission in the visible region were recorded.
DOI: 10.1021/ol1019772